A Brief Introduction to Copper Electrorefining
Overview
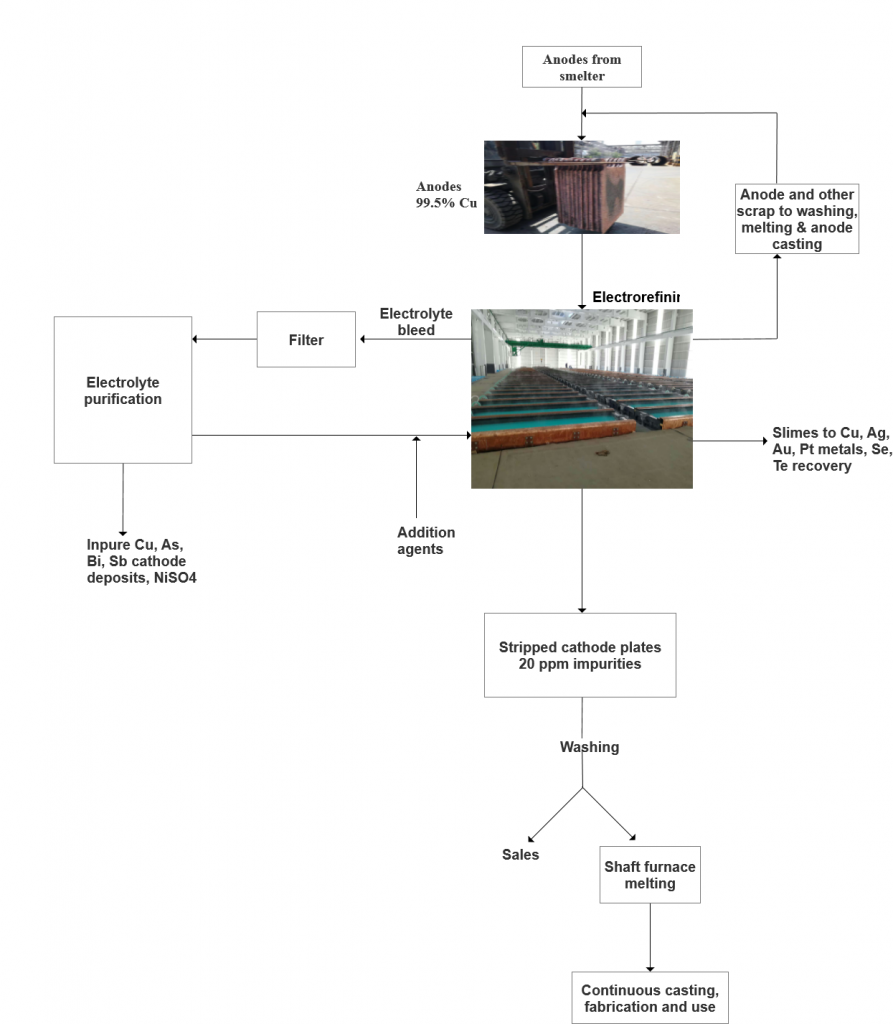
Electrolytic refining process is used to produce high-purity copper from impure copper anodes. The process involves electrochemical dissolution of copper from anodes and selective electroplating of pure copper onto cathodes.
Electrolytic Cell Setup
— The process takes place in an electrolytic cell filled with an electrolyte solution containing CuSO4 (copper sulfate) and H2SO4 (sulfuric acid).
— Copper anodes (impure copper) and cathodes (usually made of stainless steel) are immersed in the electrolyte, with a typical anode-cathode distance of approximately 50 mm.
Electrochemical Reactions
–— Anode Reaction: Copper from the anode is electrochemically oxidized and dissolves into the electrolyte as Cu2+ ions, releasing electrons:
Cu (anode) → Cu2+ (in electrolyte) + 2e–
–— Cathode Reaction: Copper ions in the electrolyte are reduced and plated onto the cathode as pure copper, using the electrons supplied through an external circuit:
Cu2+ (in electrolyte) + 2e– → Cu (cathode)
Impurity Management
— Insoluble impurities in the anode, such as gold, silver, and platinum group metals, form a slime layer that adheres to the anode or falls to the bottom of the cell.
— Soluble impurities like arsenic (As), antimony (Sb), bismuth (Bi), iron (Fe), nickel (Ni), and sulfur (S) are continuously removed from the electrolyte through a bleed stream to prevent contamination of the cathode copper.
Electrolyte Management
— The electrolyte is heated to 60-65°C to improve its electrical conductivity and mass transfer.
— It is continuously circulated at a rate of approximately 1.2 m³/h to ensure uniform concentrations of Cu2+ ions and additives across all cathode surfaces.
Additive Agents
–— Leveling Agents: Protein colloids (e.g., bone glue) are added to promote the deposition of a smooth, dense copper layer on the cathode.
–— Grain-Refining Agents: Thiourea and chloride ions are used to refine the grain structure of the deposited copper, leading to a high-quality cathode surface.
Operational Parameters
— The applied cell voltage is typically around 0.3 V, which includes overvoltages for copper plating on the cathode and dissolution from the anode.
— Current density, measured in amperes per square meter (A/m²), is a critical parameter that affects the rate of copper deposition and the quality of the cathode.
Cathode Production
— Stainless steel blanks serve as the starting cathodes onto which copper is electrodeposited for 7-10 days.
— Once the copper has been deposited, the cathodes are removed, washed, and the copper deposits are stripped off the stainless steel for further processing or shipping.
Anode Preparation
— Impure copper anodes are cast with a typical purity of 98.5-99.5% Cu and are prepared to ensure uniform dissolution during the refining process.
— Anode scrap, once removed from the cell, is washed, dried, remelted, and recast into new anodes.
Environmental and Energy Considerations
— Energy consumption is minimized through efficient electrolyte heating, cell insulation, and energy-efficient melting of anode scrap.
— Byproduct metals are recovered from the slimes for economic and environmental benefits.
Monitoring and Control
— The refining process is monitored using infrared scanners, gauss meters, and cell voltage monitoring systems to detect and rectify short circuits and ensure optimal operation.
— Online measurement systems are employed for real-time monitoring of electrolyte parameters such as temperature, voltage, and additive concentrations.
Recent Developments and Trends
— There is a trend towards increased adoption of polymer concrete cells and permanent cathode systems, especially in regions with high labor costs.
— Automation, robotics, and advanced process control technologies are becoming standard in the industry to improve efficiency, safety, and productivity.
— Efforts are being made to treat anodes with more complex impurity profiles at higher current densities while maintaining high cathode purity.







 2024-06-20 14:17:50
2024-06-20 14:17:50